Do Patients Actually Choose Authorized Generics? The Real Story Behind the Labels

When you pick up a prescription, you probably don’t think about whether the pill in your hand is the brand name or a generic. But there’s a big difference between the generics you see on the shelf-and one of them might surprise you. Authorized generics aren’t like the usual cheap generics you’ve heard about. They’re made by the same company that makes the brand-name drug, in the same factory, with the exact same ingredients. And yes, people notice.
What Exactly Is an Authorized Generic?
An authorized generic is the brand-name drug, but sold without the brand name on the bottle. It’s not a copy. It’s the same pill, same capsule, same coating, same inactive ingredients-everything. The only difference? The label says ‘generic’ instead of ‘Lipitor’ or ‘Prozac.’
Here’s how it works: A company like Pfizer makes a drug called Lipitor. When the patent expires, another company can make a cheaper version through the regular generic approval process. But Pfizer can also make its own version of Lipitor and sell it under a generic label. That’s an authorized generic. It doesn’t need to go through the usual FDA review because it’s already approved under the original brand’s application. That means it hits the market faster than traditional generics.
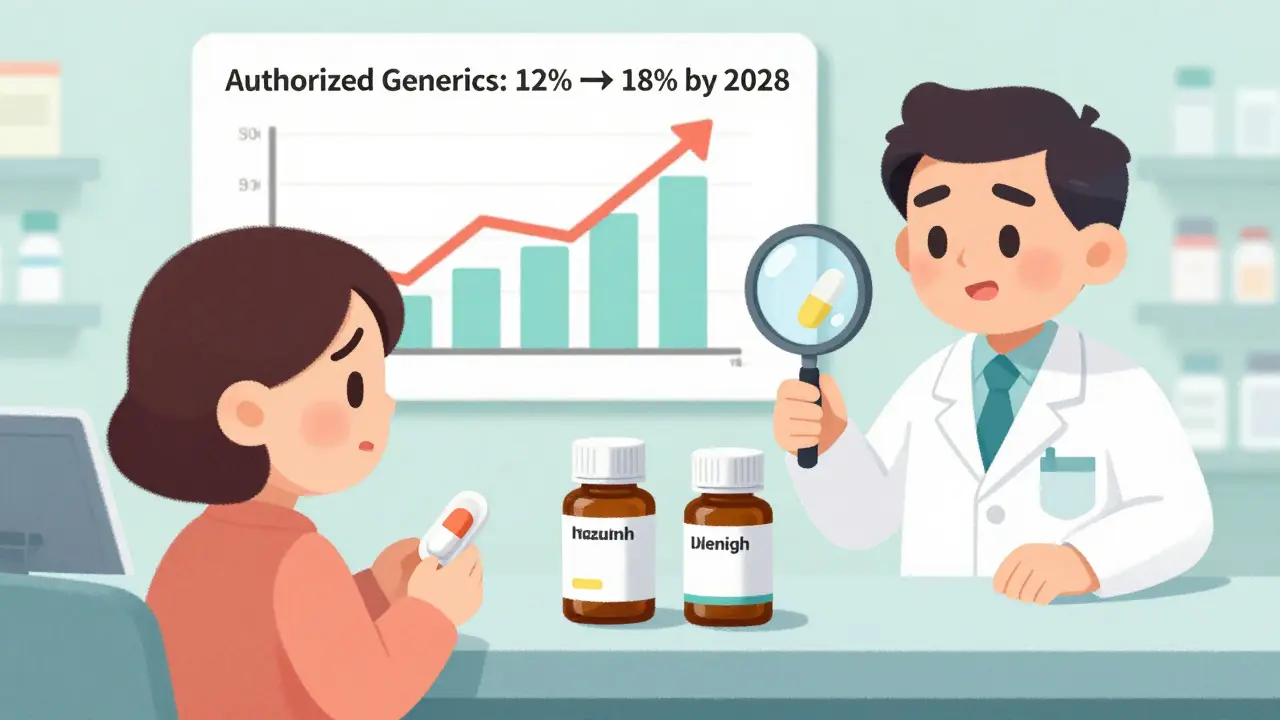
This isn’t new. The FDA has tracked them since the early 2000s. Today, about 12% of all generic prescriptions filled in the U.S. are authorized generics. That’s up from 8% just ten years ago.
Why Do Patients Stick With Authorized Generics?
Most people don’t care about the paperwork behind their medicine. They care about how it works-and whether they feel different after switching.
A 2018 study tracking over 210,000 patients found something surprising: when people switched from a brand-name drug to an authorized generic, only 22.3% went back to the brand. That’s compared to 28.7% who switched back after trying a traditional generic. That’s a 22% drop in switchbacks. Why? Because they didn’t notice any difference.
Patients aren’t just guessing. In a Consumer Reports survey, 78% of people couldn’t tell the difference between an authorized generic and the brand-name drug when given unlabeled pills. That’s way higher than the 52% who couldn’t tell the difference with regular generics. That’s because traditional generics can have different fillers, dyes, or coatings. Sometimes those changes cause side effects-stomach upset, dizziness, or even allergic reactions. Authorized generics don’t have those issues because they’re identical.
One Reddit user wrote: ‘I’ve been on the same antidepressant for five years. Switched to the authorized generic and didn’t feel a thing. The regular generic? I got headaches for two weeks.’ That’s not rare. Pharmacists in Texas and Ohio told me they see it all the time: patients who refuse traditional generics because they ‘don’t work like the brand.’ But they’ll take the authorized one without question.
But What About Price?
Here’s the catch: authorized generics aren’t always the cheapest option.
When a new generic hits the market, the authorized generic usually comes in first. During the first 180 days after a brand loses patent protection, prices drop 4% to 14% if an authorized generic is there. That’s helpful. But after that, traditional generics-made by companies like Teva or Mylan-come in at 15% to 25% cheaper.
So what do people do? They switch. Once the price gap widens, cost wins. In the second half of the generic competition window, traditional generics take over 65% of the market. Authorized generics drop to 20% or less. People don’t hate them. They just don’t pay more for something that’s the same if a cheaper version exists.
One Medicare beneficiary in Ohio told me: ‘I’ll take the authorized generic if it’s the same price as the other one. But if the other one is $5 and this one is $12? I’m taking the $5.’
Who Decides What You Get?
Here’s the hard truth: most patients don’t get to choose.
Insurance plans and pharmacy benefit managers (PBMs) decide which drugs are on the formulary. In 82% of commercial insurance plans, the pharmacy automatically fills a generic version-no questions asked. The only way you get the brand name is if your doctor writes ‘Do Not Substitute’ on the prescription.
Even then, you might not get the one you want. If your plan covers both an authorized generic and a traditional generic, they’ll usually pick the cheapest one. Sometimes, the pharmacy doesn’t even know which is which. Authorized generics show up in the FDA’s Orange Book under ‘Products with No Applicant’-a confusing label even for pharmacists.
That’s why some pharmacists now carry two bottles of the same drug: one labeled ‘Lipitor’ and one labeled ‘atorvastatin.’ They have to explain the difference to patients who think they’re getting scammed. ‘It’s the same pill,’ they say. ‘Just a different label.’
Why Do Drug Companies Use Authorized Generics?
It’s not just about helping patients. It’s about business.
When a brand loses patent protection, it’s a financial freefall. Sales can drop 80% in a year. Authorized generics let the brand company stay in the game. They keep making money, keep their workforce, and keep their factory running. And they can undercut new generic competitors before they even get started.
The Federal Trade Commission has raised red flags. In some cases, brand companies have agreed with generic manufacturers: ‘You don’t launch your generic, and we won’t launch ours.’ That’s called a ‘pay-for-delay’ deal. It’s not illegal, but it’s controversial. The FTC says these deals cost consumers billions.
But here’s the flip side: if a brand company launches an authorized generic, prices drop faster. Medicaid data on one heart medication showed prices fell 18% faster when an authorized generic entered the market. For people on fixed incomes, that matters.
What’s the Future?
More brands are using authorized generics. Seven of the top ten drugmakers now have at least one on the market. Cardiovascular and mental health drugs lead the way-exactly the kinds of medications where patients are most sensitive to changes.
The FDA is trying to make it easier to spot them. In 2023, they started updating a public list of authorized generics every month. But most people still don’t know how to find them.
Experts predict authorized generics will make up 15% to 18% of all generic prescriptions by 2028. But if drugmakers use them to delay cheaper competition, Medicare could pay $1.2 billion more over the next few years.
What Should You Do?
If you’re on a brand-name drug and you’re worried about cost or side effects:
- Ask your pharmacist: ‘Is there an authorized generic for this?’
- Check the label. If it says the same active ingredient as your brand, and the manufacturer is the same (like Pfizer or Merck), it’s likely an authorized generic.
- Compare prices. If the authorized generic costs the same as the traditional one, take it. You’re getting the same drug.
- If the traditional generic is much cheaper, go with that. You’re not losing anything.
- If you feel worse after switching, tell your doctor. It might not be the drug-it might be the fillers.
You don’t need to be a pharmacist to make smart choices. You just need to ask the right questions. And sometimes, the best version of your medicine isn’t the one with the fancy name on the bottle-it’s the one with the same formula, and no extra cost.
What’s the Bottom Line?
Patients don’t choose authorized generics because they’re trendy. They choose them because they work the same way-and they don’t make them feel weird. The data proves it: fewer people switch back. Fewer side effects. More consistency.
But money still rules. Once the price drops low enough, people go for the cheapest option. That’s not a flaw. That’s human behavior.
Authorized generics aren’t a scam. They’re not magic. They’re just another tool in a broken system. And for the people who need stability in their meds? They’re the closest thing to a win.
Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are made by the same company as the brand-name drug, in the same factory, using the exact same ingredients-active and inactive. The only difference is the label. The FDA confirms they are chemically identical.
Why do some people feel different on traditional generics?
Traditional generics must meet bioequivalence standards, but they can use different fillers, dyes, or coatings. For some people, especially those with sensitivities, these differences cause side effects like nausea, headaches, or mood changes. Authorized generics avoid this because they’re identical to the brand.
Can I ask my pharmacy for an authorized generic?
You can ask, but your insurance may not cover it if a cheaper traditional generic is available. Some pharmacies won’t even know which version they’re dispensing unless you specifically request it. Ask your pharmacist to check the manufacturer and label.
Are authorized generics more expensive than regular generics?
Usually, yes-at least at first. Authorized generics enter the market right after patent expiry and are priced closer to the brand. Traditional generics drop lower after 180 days. But if the prices are the same, the authorized version is the better choice because it’s identical to the brand.
How do I know if my prescription is an authorized generic?
Check the manufacturer name on the bottle. If it matches the brand-name drug’s maker (e.g., Pfizer for Lipitor), it’s likely an authorized generic. You can also ask your pharmacist or look up the drug in the FDA’s ‘Products with No Applicant’ list online.

9 Comments
This whole thing is a scam wrapped in a lab coat. Big Pharma doesn't care if you feel better-they care if you keep paying. Authorized generics? More like 'authorized profit protection.' They delay real competition so they can keep jacking up prices until the cheap generics show up. And now they're acting like heroes? Please.
It's fascinating how we've built an entire pharmaceutical ecosystem around the illusion of choice. The patient believes they're selecting based on efficacy or cost, but in reality, their agency is mediated by insurance algorithms, opaque formularies, and corporate strategy disguised as consumer benefit. The authorized generic isn't a triumph of transparency-it's a sophisticated maneuver in a game where the house always wins, and the patient is just another variable in the cost-benefit equation. We praise efficiency while ignoring the structural coercion that makes it possible.
I switched to an authorized generic for my anxiety med last year and honestly? No difference. Zero. My pharmacist even had to explain it to me because the bottle looked totally different. I was nervous at first-heard horror stories about generics making people feel off-but this one? Same pill, same results. I wish more people knew this was an option. It’s not magic, it’s just… science.
Same pill, different label. Why are we making this complicated?
So let me get this straight-Big Pharma makes the exact same drug, slaps a generic label on it, and suddenly they're the good guys? Meanwhile, the real generics get painted as 'unsafe' while the authorized ones get a free pass? Classic. The only thing that changed is the label and the profit margin. I'm not buying the 'patient-first' narrative. This is capitalism with a nice smile.
Authorized generics, by definition, are bioequivalent to the reference listed drug and are manufactured under the same NDA. Their pharmacokinetic parameters fall within the FDA’s 80–125% confidence interval for bioequivalence, which is identical to traditional generics. The perceived clinical difference is likely attributable to the nocebo effect, compounded by variability in inactive ingredients in non-authorized generics. The data cited in the post is methodologically sound and corroborated by multiple cohort studies.
Why are we even talking about this like its a big deal? In India we have generics that cost 10x less and work just fine. You guys overcomplicate everything. If the pill works dont complain. Stop crying about labels
So… I pay more for the same pill because the label says 'Pfizer' instead of 'Teva'? Got it. Next you’ll tell me the color of the pill matters.
I’ve been telling my friends this for years. My mom’s blood pressure med? Switched to the authorized generic and her dizziness disappeared. The other generic made her feel like she was drunk. I don’t care what it’s called-I care that she’s not falling over. Ask your pharmacist. It’s not that hard.