Multiple Myeloma: Understanding Bone Disease and the New Drugs Changing Outcomes
Over 80% of people diagnosed with multiple myeloma will develop severe bone damage. It’s not just a side effect-it’s a core part of the disease. These aren’t ordinary bone weak spots. They’re osteolytic lesions, holes in the bone that look like they’ve been punched out on an X-ray. And they don’t just hurt-they break. Fractures happen without falls. Spinal cord compression can paralyze. Calcium floods the bloodstream, causing confusion and kidney failure. For many, the bone damage is what brings them to the hospital, not the cancer itself.
Why Does Myeloma Eat Your Bones?
Your bones aren’t just static scaffolding. They’re alive, constantly being broken down and rebuilt. Osteoclasts chew away old bone. Osteoblasts build new bone. In healthy people, these two work in balance. In multiple myeloma, that balance shatters.
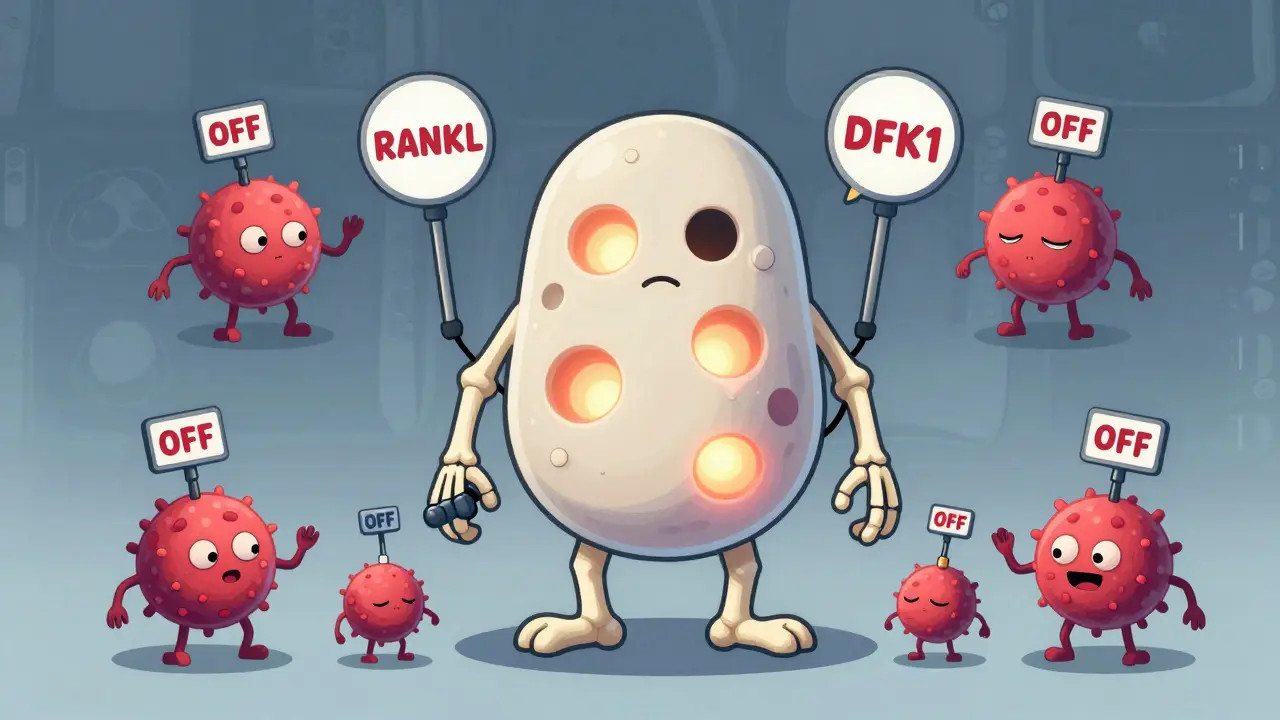
Myeloma cells don’t just sit in the bone marrow-they actively hijack the system. They flood the area with signals that turn osteoclasts into overachievers. At the same time, they shut down osteoblasts, stopping bone repair. One key player is RANKL, a protein myeloma cells force bone cells to make. RANKL acts like a gas pedal for osteoclasts. Meanwhile, myeloma cells pump out DKK1 and sclerostin, proteins that block the Wnt pathway-the body’s main signal for building bone. Without Wnt, osteoblasts can’t do their job.
Even the osteocytes, the most common bone cells, get dragged into the chaos. They start releasing more sclerostin, worsening the shutdown of bone formation. Studies show myeloma patients have sclerostin levels nearly 50% higher than healthy people. And the more DKK1 in the blood, the worse the bone damage. Patients with levels above 48.3 pmol/L have over three times as many bone lesions.
The Vicious Cycle No One Talks About
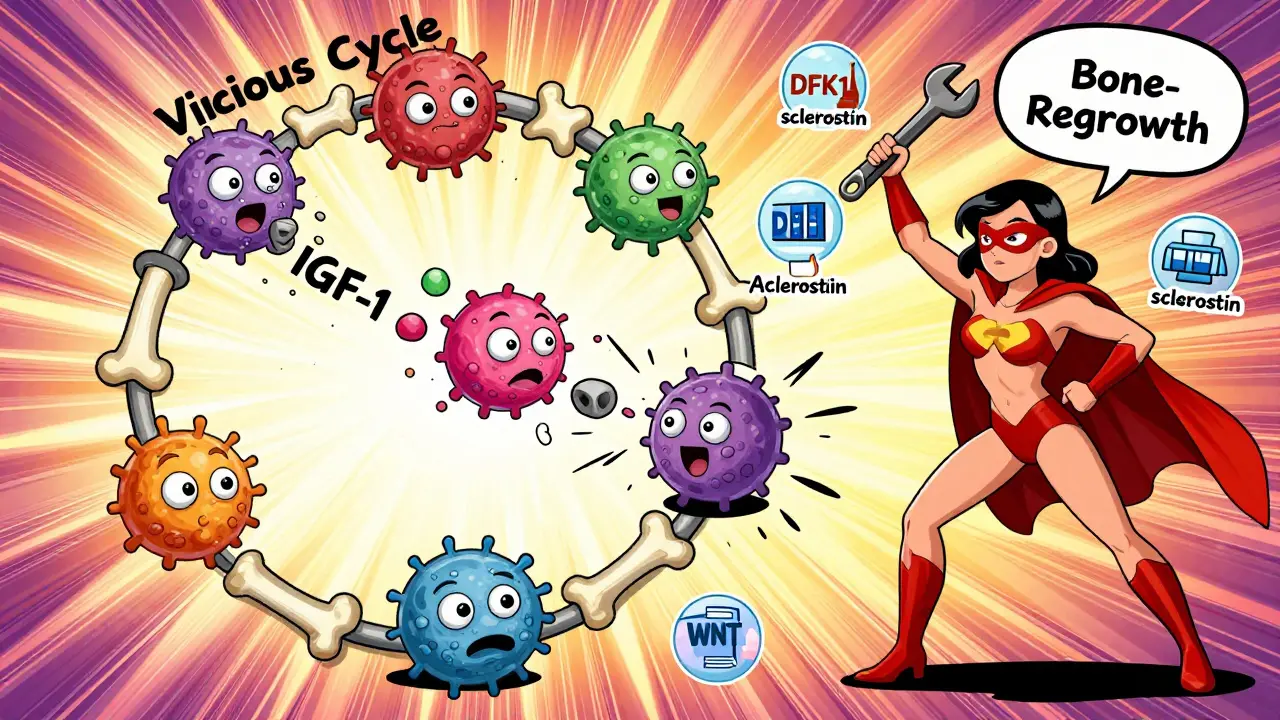
Here’s the cruel twist: when bone breaks down, it releases growth factors trapped in the bone matrix-things like IGF-1 and TGF-beta. These aren’t just waste products. They’re food for myeloma cells. So as the bone gets destroyed, the cancer grows stronger. And as the cancer grows, it makes even more signals to destroy more bone. It’s a loop with no easy exit.
Experts call it the "vicious cycle." Dr. Evan Winter from Mayo Clinic puts it plainly: "The bone isn’t just a victim. It’s an active partner in the disease." That’s why treating just the cancer cells isn’t enough. You have to break the cycle at both ends.
Current Standard Care: Bisphosphonates and Denosumab
For over two decades, the go-to treatment has been bisphosphonates-zoledronic acid or pamidronate. Given as monthly IV infusions, they stick to bone surfaces and kill osteoclasts. They reduce fractures by about 15-18% compared to no treatment. But they come with trade-offs. About 27% of patients develop kidney problems. One in five need dose changes because their creatinine clearance drops below 60 mL/min. And about 1 in 10 get osteonecrosis of the jaw-a rare but serious condition where the jawbone starts dying after dental work.
Denosumab, approved in 2010, is a different kind of drug. Instead of targeting osteoclasts directly, it blocks RANKL-the signal that activates them. It’s given as a monthly shot under the skin. No IV. No kidney strain. In one Mayo Clinic study, 74% of patients preferred it over IV therapy. But it’s expensive: $1,800 per dose versus $150 for generic zoledronic acid. And it carries the same jawbone risk. Still, for patients with kidney issues, it’s often the only option.

The New Wave: Drugs That Don’t Just Slow Damage-They Heal
The real breakthrough isn’t just stopping bone loss. It’s making bone grow back.
Anti-sclerostin drugs like romosozumab and blosozumab are the most promising. They block sclerostin, lifting the brake on bone formation. In a 2021 trial with 49 myeloma patients, romosozumab increased bone density in the spine by 53% in just 12 months. That’s more than most osteoporosis drugs achieve in two years. Patients also reported a 35% drop in bone pain.
Then there’s DKN-01, an anti-DKK1 antibody. In a 2020 trial of 32 patients, it cut bone resorption markers by 38%. Early data suggests it may even help restore some bone structure-not just prevent more damage.
Other targets are in the pipeline. Gamma-secretase inhibitors like nirogacestat block the Notch pathway, which myeloma cells use to boost RANKL. In lab models, they cut bone lesions by 62%. RNA therapies are also being tested. Alnylam’s ALN-DKK1 reduced DKK1 levels by 65% in preclinical studies.
But here’s the catch: none of these have yet proven they extend life. Dr. Kenneth Anderson from Dana-Farber warns, "We’re seeing great bone markers, but we still don’t know if they translate to longer survival." That’s why they’re not standard yet. They’re still in trials.
What Patients Are Really Experiencing
On Reddit’s r/myeloma community, a 2023 thread with 147 comments revealed a pattern: 68% still had bone pain despite being on bisphosphonates. Many said their doctors didn’t take the pain seriously until a fracture happened. One patient wrote, "I had three vertebral fractures before they agreed to switch me to denosumab. By then, I was in a wheelchair."
Another common complaint: dental issues. Because of MRONJ risk, patients need a dental checkup within 30 days of starting any bone drug. Many delay it because they’re overwhelmed. The International Myeloma Foundation’s 2022 survey found bone complications led to hospitalization for 32.7% of patients-second only to infections. Average stay? Over eight days.
But those in novel agent trials report a different story. One participant in the romosozumab trial said, "For the first time since diagnosis, I slept through the night. No painkillers. Just… quiet."
Who Gets What, and Why?
Guidelines from the International Myeloma Working Group (IMWG) say every newly diagnosed patient needs a full bone scan-either a whole-body low-dose CT or PET-CT. No exceptions.
For treatment, most U.S. doctors start with denosumab if kidney function is normal. If not, they go with zoledronic acid. But cost and access matter. In Europe, only 42% of patients get denosumab because insurance often won’t cover it. In Asia, bisphosphonates still dominate at 89% usage.
Novel agents aren’t available outside trials yet. But that’s changing fast. The FDA approved a new low-dose version of zoledronic acid in April 2023-Zometa-LD-with less kidney toxicity. And the phase III BONE-HEAL trial, testing romosozumab in 450 patients, is now enrolling.
The Future: Healing, Not Just Preventing
By 2030, experts believe we’ll stop talking about "managing bone disease" and start talking about "healing bone." The goal isn’t just to prevent fractures. It’s to rebuild them. Bispecific antibodies that target both myeloma cells and bone signals are already in early trials. One candidate binds to BCMA (a myeloma marker) and CD3 (a T-cell activator), killing cancer while also reducing RANKL.
Personalized medicine is coming too. Doctors may soon check blood levels of DKK1, sclerostin, and bone turnover markers to pick the right drug for the right patient. Someone with high sclerostin? Anti-sclerostin therapy. Someone with high DKK1? DKN-01. It’s not science fiction-it’s happening in labs right now.
Dr. Brian Durie of the International Myeloma Foundation says it best: "We’re moving from protecting bones to restoring them. In ten years, bone disease won’t be the reason people die of myeloma. It’ll be the thing we fixed."
What You Need to Do Now
If you or someone you know has multiple myeloma:
- Get a full bone scan at diagnosis-don’t wait for pain.
- Ask about kidney function before starting any bone drug.
- See a dentist within 30 days of starting treatment.
- Track your bone pain. If it’s not improving, ask if you’re a candidate for denosumab or a clinical trial.
- Don’t assume standard care is the only option. Novel agents are coming fast.
The landscape has changed. You don’t have to live with broken bones. There are better tools now. And even better ones are on the way.
Can multiple myeloma bone damage be reversed?
Yes, in some cases. Current drugs like bisphosphonates and denosumab stop further damage but don’t rebuild bone. Newer agents-like romosozumab and anti-DKK1 therapies-have shown in trials that they can actually increase bone density and repair lesions. In one study, patients saw a 53% increase in spinal bone density within a year. While full reversal isn’t guaranteed for everyone, healing is now possible, not just prevention.
Is denosumab better than zoledronic acid?
It depends. Denosumab is more convenient (monthly shot vs. IV infusion), easier on the kidneys, and preferred by most patients. But it’s much more expensive-around $1,800 per dose versus $150 for generic zoledronic acid. If kidney function is poor, denosumab is the safer choice. If cost is a barrier, zoledronic acid still works well. Both reduce fractures by about the same amount. The choice often comes down to access, cost, and kidney health.
What are the biggest side effects of bone drugs for myeloma?
The main risks are osteonecrosis of the jaw (MRONJ), kidney damage, and low calcium. MRONJ happens in up to 10% of patients on long-term therapy and often requires dental surgery. Zoledronic acid can lower kidney function, especially in older adults. Denosumab causes low calcium in about 12% of patients, so calcium and vitamin D supplements are required. Newer drugs like romosozumab also carry a hypocalcemia risk, requiring monthly blood checks.
Why do I need a dental checkup before starting bone treatment?
Because the drugs that stop bone loss can also stop bone healing. If you have an infected tooth or need a tooth pulled while on these drugs, your jawbone may not heal properly, leading to osteonecrosis. A dental exam before starting treatment lets your dentist fix problems ahead of time. Most guidelines say this checkup should happen within 30 days of starting therapy.
Are there any new drugs for myeloma bone disease coming soon?
Yes. Romosozumab is in a large phase III trial called BONE-HEAL, with results expected by 2027. Anti-DKK1 drugs like DKN-01 are in mid-stage trials. Bispecific antibodies that target both myeloma cells and bone signals are in early testing. RNA therapies that silence DKK1 production are also advancing. These aren’t available yet, but many are expected to reach patients within the next 3-5 years.

15 Comments
This is the most important thing I've read all year. We're not just treating cancer anymore-we're rebuilding bones. If you're on bisphosphonates and still in pain, you're being failed. Demand denosumab. Demand trials. Your spine doesn't deserve to be Swiss cheese.
Bro, this ain't just science-it's poetry. Osteoclasts as overachievers? Sclerostin as a silent assassin? DKK1 as the ghost in the machine? Myeloma didn't just steal my bones, it turned my marrow into a warzone with traitors on both sides. But now? Now we got generals who know how to flip the script. Romosozumab? That's not a drug-it's a revolution wrapped in a syringe.
I cried reading this. My dad had three spinal fractures before they switched him to denosumab. He slept through the night for the first time in 2 years last week. 💙
Of course they're pushing these expensive drugs. Pharma doesn't care if you live or die-they care if your insurance pays. Denosumab costs $1,800? That's not medicine, that's a luxury subscription. And don't get me started on the jaw necrosis. They'll amputate your face to sell you a miracle.
The claim that "bone damage can be reversed" is misleading. The study cited showed increased bone density, not radiographic healing of lytic lesions. Density ≠ structural integrity. Until there's histological evidence of lesion closure and biomechanical restoration, this is hype masquerading as science. Also, "53% increase in spinal density"-in what population? Controls? Baseline? p-values? No data transparency, no credibility.
Sclerostin up 50 percent DKK1 over 48 pmol L leads to 3x lesions RANKL is the gas pedal Wnt is the brake bisphosphonates kill osteoclasts denosumab blocks RANKL romosozumab blocks sclerostin DKN01 blocks DKK1 you dont need to understand biology you need to understand the numbers
Wow. I didn't know bone could heal like this. I thought it was just "stop it from getting worse." This gives me so much hope. I'm gonna ask my oncologist about the trial next week. Fingers crossed 🤞
They say these drugs are "new" but what if this is all a distraction? What if the real cause is glyphosate in the water? Or 5G messing with bone cell signaling? They don't want you to know the truth. They're selling you expensive injections while the real villains-Big Pharma and the CDC-are laughing all the way to the bank. And don't get me started on the dental checkup requirement. That's not prevention-it's control.
Let me tell you something about bone remodeling. It's not just about blocking signals. It's about the microenvironment. The hypoxic niche in the marrow. The exosomes myeloma cells pump out. The way mesenchymal stem cells get reprogrammed into accomplices. We've been looking at this like it's a simple on-off switch but it's a symphony of failure. And now we're finally tuning the instruments. Romosozumab doesn't just lift the brake on osteoblasts-it reawakens them. That's not just healing. That's resurrection. And if you think this is expensive now, wait till the patents expire. This is the future, and it's cheaper than a lifetime of wheelchairs and morphine.
Why are we even talking about this? America spends billions on drugs while people can't afford insulin. This is just another way to profit off sick people. And don't even get me started on how they're pushing this as a "breakthrough" when we still don't have a cure. Wake up. This is capitalism in action.
They say "healing bone" but they never mention the 12% of patients who get severe hypocalcemia from romosozumab. Or how the phase III trial excludes anyone over 75. Or that the only data we have is from 49 patients. This isn't medicine. It's a gamble with your spine. And the doctors? They're just cheerleaders for the next big thing.
Bro I just got off the phone with my oncologist and we're switching me to denosumab next week. Been on zoledronic acid for 18 months and my pain hasn't budged. If this thing lets me walk without a cane again I'm gonna hug the nurse. Thanks for the heads up on the trial info-going to look into it right now.
There's something beautiful here. The body doesn't just want to survive-it wants to rebuild. Myeloma tried to turn bone into dust, but science is teaching it that healing is stronger than destruction. We're not just fighting cancer anymore. We're helping bones remember how to be bones. That's not just medical progress. That's spiritual. And if you're reading this and you're scared? You're not alone. But you're also not powerless. There's light in the marrow now.
How dare you suggest patients should "ask about trials"? You're putting pressure on people who are already broken. What if they can't afford it? What if they're too tired? What if they're scared? This isn't a checklist. This is someone's life. Stop treating people like data points.
I just wanted to say thank you for writing this. My mom started romosozumab last month and her pain level dropped from 8/10 to 3/10 in two weeks. We're not celebrating yet-but we're breathing again. And for the first time, we feel like we're not just waiting for the next fracture.